Keywords: target, release time, isotope.
Introduction
The development of rapid and chemically selective targets is of great importance for any ISOL-facility. The release of nuclides proceeds in two subsequent steps: diffusion from the target and desorbtion from the surface. The speed of these processes exponentially depends on temperature, therefore it is preferably to use the most thermostable substances as a target material. A lot of elements form chemical compounds with the carbon which are stable at very high temperatures. The most wide-spread high temperature carbon compounds are carbides. They are commonly used as a target material for production of many far from stability nuclei [1,2]. Another kind of carbon substances - the product of pyrolysis of the diphtalocyanine compounds (DPC) of the III-IV group elements of Periodic Table - was developed and is commonly used at IRIS facility for production of radioactive nuclei [3]. Since 1980 isotopes of eleven elements have been studied with the aid of this type of target [4-6].
The molecule of DPC consist of two large organic planes between which the atom of the metal is placed. The structure of pyrolysis product was not thoroughly studied. It was found by means of X-ray spectrography and neutron diffraction analysis that there are no chemical Me-C bonds and the structure of DPC compound is amorphous. So DPC compounds and metal carbides are rather different chemical substances.
2. Technology of preparation
The preparation of targets materials is fulfilled via diphtalocianine synthesis. The technology of preparation of DPC compounds is quite different for III group metals (including REE) and for IV-VI groups metals.
2.1 Preparation of DPC of U, Th and Zr.
ii) Metal powder is converted into respective iodine with I2 vapor. Iodine crystals are placed in a quartz boat which is placed in the reaction tube whilst metal is kept under Ar stream. By raising the iodine bath temperature to 120 -140 oC, a partial pressure of iodine of 100 -200 mm of Hg can be achieved, enabling MeI 4 to be formed.
iii) The metal iodine is transferred from the reaction vessel and placed in the molten dicyanobenzen under an Ar stream and heated to 250oC for 30 min.
This product can be obtained by much more simple way. The corresponding DPC are formed via reaction of metal acetate with dicyanobenzen by the same condition as for uranium (see 2.1)
2.3 Pyrolysis and further operations.
The pyrolysis takes place in vacuum or under the
Ar atmosphere at a temperature 600-700oC which is then raised
slowly up to 11000C.A carbon-like, highly porous, brittle and
hard material in a form of granula is produced as a result of pyrolysis.
The granula are pounded and sieved through a 0.08 mm sieve. The powder
is put into a tantalum vacuum oven and trained by increasing the temperature
up to the value of 2200-2300oC at high vacuum. The experimental
set up ( a target construction etc.) is described in detail in [3].
The main features of the pyrolised DPC's as a target
material, the advantages and disadvantages in comparison with the carbide
targets as well as the construction of the target-ion source device at
the IRIS facility are described in detail in Ref. [7], so we consider here
only two items which have been additionally investigated: the release properties
of the target material and its sintering during a long period of exploitation.
3. The release properties
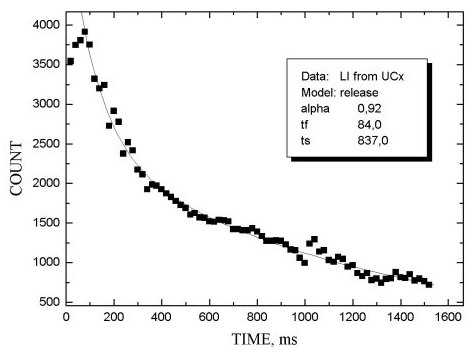
The release properties of MeCX targets were investigated by measuring the b -activity as a function of the delay time between the end of irradiation and beginning of ion beam collection. The release function was approximated by two exponents with different characteristics: the first one describes the fast process of product release, the second - slow tail of release. The corresponding time parameters are tf and tS. Parameter a shows the relative intensity of the fast process.
4. Results and discussion
In figs 1-5 release functions for Li, Na, Rb and In are presented. The parameters for fitting two-exponent functions and the type of target material are shown in the script windows on each figure. The temperature of the target was ~ 2300oC.

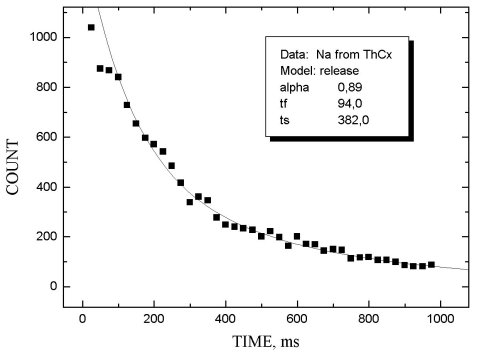
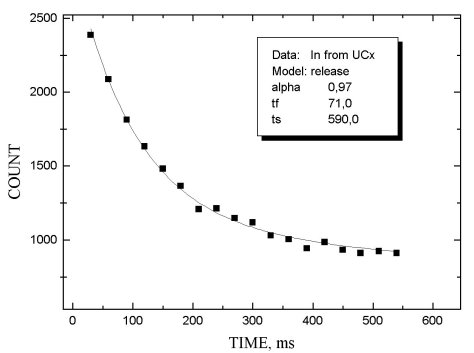
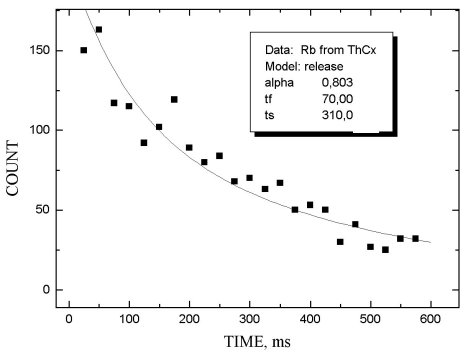
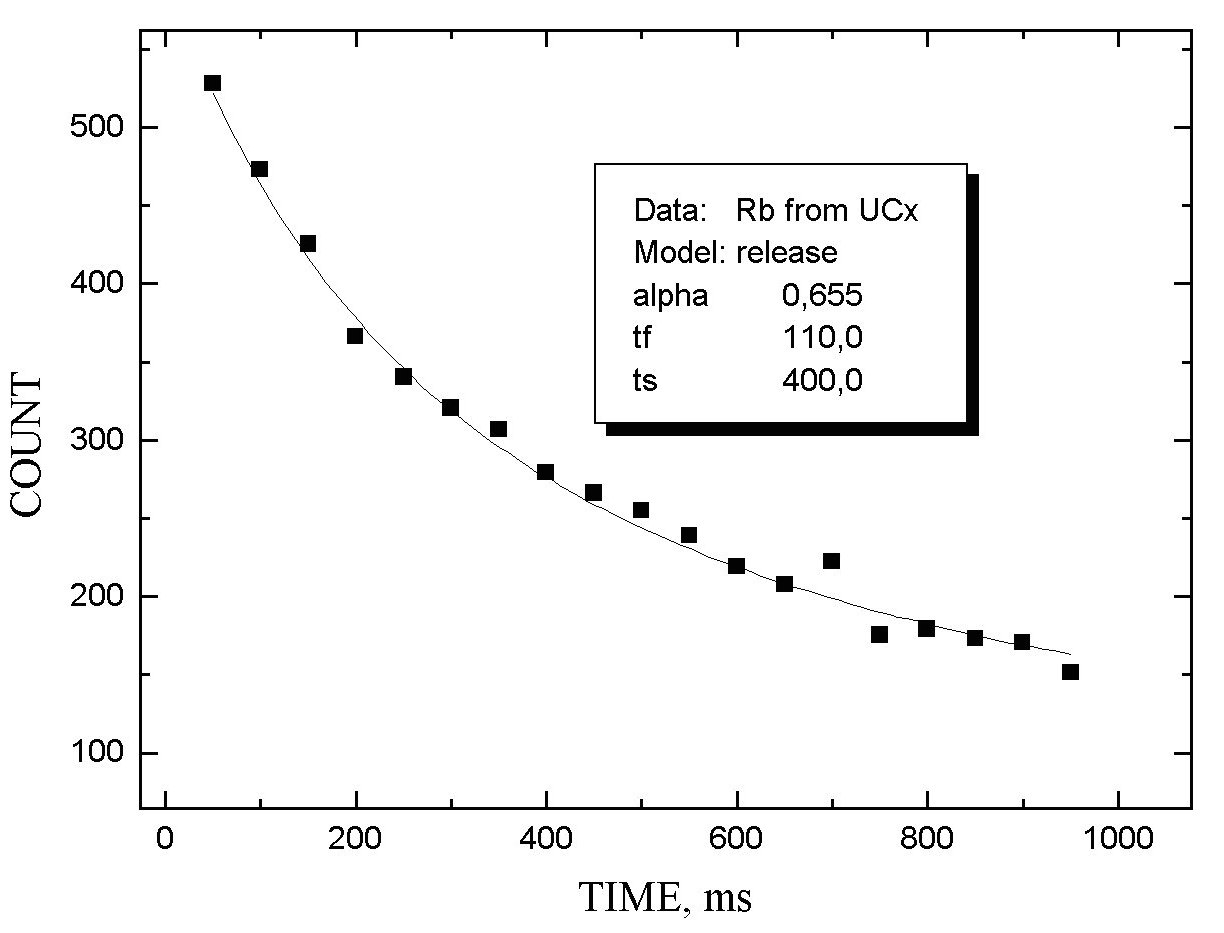
It is worth noting that there is no clear difference
between results obtained for UCX and ThCX targets
(see table 1 where data on Rb release properties for these two targets
are given). The effect is seemed to be the consequences of the specific
structure of the target materials: their thermochemical properties are
based on the same structure of carbon matrixes and do not depend on the
metal used.
| T(oC) | ThCx | UCx |
| 2000 ( ISOLDE )
2100 ( ISOLDE ) 2300 ( IRIS ) |
200 ms
30 ms 70 ms |
800 ms
- 110 ms |
The fast release of the reaction products from the
DPC targets and their universality enable one to use this type of target
in different applications where the production of the extremely short lived
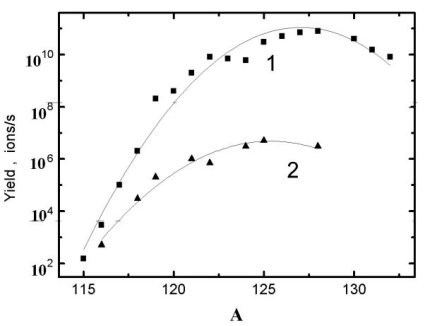
nuclei is needed. For example, in fig.6 the neutron deficient Cs isotopes
yields from the molten La target (ISOLDe) and from the GdCX
target are compared.
|
|
|
|
|
| 1. UCX
(~10 g/cm2) |
|
|
|
| 2. ThCX
(~10 g/cm2) |
Tl*-185 Tl-184 Tl*-183 Tl*-182 Tl*-181 Tl-180 |
|
|
| 3. ZrCX
(~10 g/cm2) |
|
|
|
| 4. GdCX
(~7 g/cm2) |
|
|
|
References
[1] S.Borg, J.Berström, G.-B. Holm, B.Rydberg, L.E. De Geer, G.Rudstam, B.Grapengiesser, E.Lund and L.Westgaard, Nucl. Instr. and Meth. 91 (1971) 109.
[2] H.L. Ravn, L.C. Carraz, J. Denimal, E. Kugler, M. Scarestad,
S. Sundell and L.Westgaard,
Nucl. Instr. and Meth.
139 (1976) 257.
[3] V.A. Bolshakov, A.G. Dernyatin, K.A. Mezilev, Yu. N. Novikov,
A.G. Polyakov, A.V. Popov, Yu. Ya. Sergeev and V.I. Tikhonov, Nucl.
Instr. and Meth. B70 (1992) 69.
[4] K.A. Mezilev, Yu.N. Novikov, V.N. Panteleev, A.G. Poljakov,
Yu.Ya. Sergeev and V.I. Tikhonov, in:
Proc. 37th Conf. on Nuclear
Spectroscopy and Nuclei Structure (Nauka,Leningrad, 1987) p. 87.
[5] V.A. Bolshakov, A.G. Demjatin, K.A. Mezilev, Yu.N.Novikov, A.V.
Popov, Yu.Ya. Sergeev and V.I. Tikhonov,
Inst. Phys. Conf. Ser.,
N132, Section 7, NFFS-Conf., 1992, ISBN, 0-7503-0262-3 (1993) p. 743.
[6] K.A. Mezilev, Yu.N. Novikov, A.V. Popov, Yu.Ya. Sergeev and V.I.
Tikhonov,
Z. Phys. A 337 (1990)
109.
[7] V.A. Bolshakov, A.G. Demjatin, K.A. Mezilev, Yu.N. Novikov, A.G.
Polyakov, A.V. Popov, Yu.Ya. Sergeev and V.I. Tikhonov, Nucl. Instr.
and Meth. B 70 (1992) 62.